Radiation therapy remains highly relevant today, as approximately half of all cancer patients receive it during their treatment course, and it contributes to about 40% of curative outcomes in oncology.[1-2] Its ongoing evolution, driven by advances in imaging, delivery, and understanding of tumor biology and immune modulation, continues to enhance efficacy and safety, making it a cornerstone of multidisciplinary cancer care.[7-8]
Article Summary
What is radiation therapy?
Radiation therapy is defined as the use of ionizing radiation to treat cancer by delivering targeted doses to malignant cells, aiming to destroy or inhibit their ability to proliferate while minimizing damage to surrounding healthy tissue.[1-2] The scientific principle underlying radiation therapy is the induction of DNA damage, primarily double-strand breaks, either directly or via the generation of free radicals, which leads to cell death through mechanisms such as mitotic catastrophe and apoptosis.[3-4]
Historically, radiation therapy began with the discovery of X-rays by Röntgen in 1895, followed by the use of radium and the development of early radiobiological theories such as Dessauer’s target theory and the dual radiation action model by Kellerer and Rossi, which clarified the sequential nature of DNA damage.[3,11] Over the past century, technological advances have transformed the field: from rudimentary X-ray machines to modern linear accelerators, and from conventional fractionation to highly conformal techniques like intensity-modulated radiotherapy (IMRT), stereotactic body radiotherapy (SBRT), and proton therapy.[2,6-7] These innovations have improved tumor targeting, reduced toxicity, and enabled integration with systemic therapies.
How does radiation damage cancer cells?
Radiation therapy works by delivering ionizing radiation that damages cancer cells primarily through the induction of DNA lesions, especially double-strand breaks (DSBs), which are the most lethal form of DNA damage.[12-14] This damage occurs via two principal mechanisms: direct ionization of DNA molecules and indirect effects mediated by the radiolysis of water, which generates reactive oxygen species (ROS) such as hydroxyl radicals. These ROS further attack DNA, proteins, and lipids, amplifying cellular injury.[14,15-16]
Upon exposure to ionizing radiation, cancer cells activate a complex DNA damage response (DDR) involving cell cycle arrest, DNA repair pathways (notably nonhomologous end joining and homologous recombination), and, if the damage is irreparable, cell death mechanisms such as mitotic catastrophe, apoptosis, necrosis, or senescence.[12-13,17] The clustered and complex nature of radiation-induced DNA damage, especially with high linear energy transfer (LET) modalities, makes repair challenging and increases cytotoxicity.[12,14,17]
Cancer cells are generally more susceptible to radiation than normal cells due to several factors: they often have defective DNA repair machinery, higher rates of proliferation (making them more vulnerable during radiosensitive cell cycle phases), and a hypoxic microenvironment that can modulate ROS effects and repair efficiency.[13-14,18] Additionally, tumor cells may have altered cell cycle checkpoints and genomic instability, further reducing their ability to survive radiation-induced damage.[13,18]
Radiation therapy exploits the differential vulnerability of cancer cells to DNA damage and ROS, leading to cell death through multiple pathways, while sparing normal tissue by leveraging differences in repair capacity and cell cycle dynamics.[12-18]
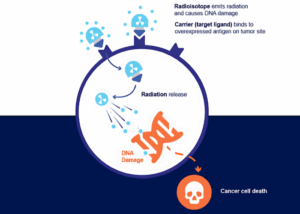
How does radiation target only cancer cells?
Radiation therapy specifically targets cancer cells while minimizing damage to surrounding healthy tissue through a multi-step process that integrates advanced imaging, personalized planning, and precision delivery. This approach is essential in modern oncology, as it maximizes tumor control and reduces toxicity, leveraging technological advances in imaging and artificial intelligence to improve outcomes.[20–23]
- First, high-resolution imaging, most commonly computed tomography (CT), but also magnetic resonance imaging (MRI) and positron emission tomography (PET), is used to precisely localize the tumor and characterize its extent and biological activity. These modalities are often fused to provide complementary anatomical and functional information, enabling accurate delineation of the gross tumor volume (GTV), clinical target volume (CTV), and planning target volume (PTV).[21,24-25] For example, PET/CT can distinguish metabolically active tumor from atelectatic lung or post-treatment changes, while MRI offers superior soft tissue contrast for head and neck or CNS tumors.[25-26]
- The medical team, including radiation oncologists, radiologists, and medical physicists, then develops a personalized treatment plan. This involves contouring the tumor and organs at risk, setting dose constraints, and using inverse planning algorithms to optimize dose distribution. Intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT) employ dynamic multileaf collimators to shape and modulate the radiation beams, conforming the high-dose region to the tumor while sparing adjacent normal tissues.[22] Daily image guidance (IGRT) and adaptive radiotherapy (ART) further refine targeting by accounting for anatomical changes and organ motion.[23,26]
Artificial intelligence-based contouring software now plays a critical role in automating and improving the accuracy of tumor and organ segmentation, reducing interobserver variability and expediting the planning process.[22-23,27] For instance, AI-driven segmentation can rapidly generate precise contours from multimodal imaging, enabling dose painting strategies that escalate dose to radioresistant tumor subregions identified by PET or MRI biomarkers.[24,27]
The integration of advanced imaging, personalized planning, precision beam shaping, and AI-driven automation enables radiation therapy to deliver curative doses to tumors while minimizing collateral damage to healthy tissue, exemplifying the principles of precision medicine in oncology.[20-23,25-27]
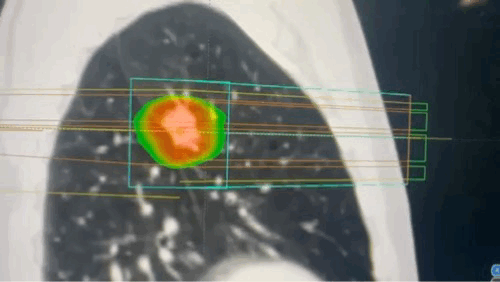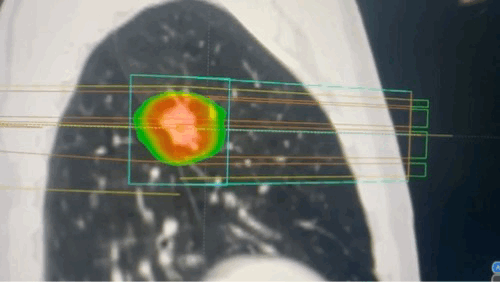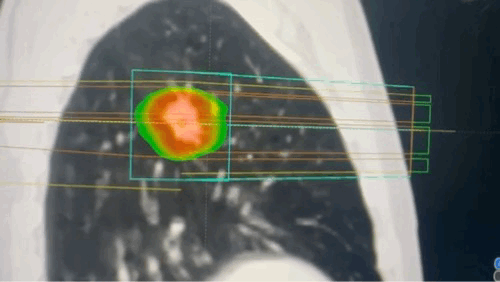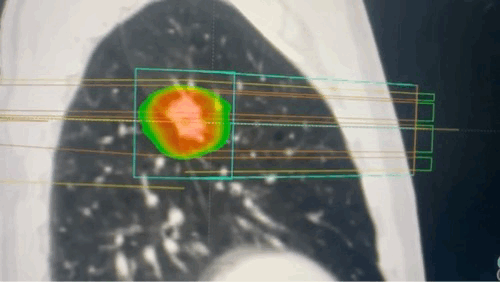
Techniques that make targeting more precise
Advanced techniques such as intensity-modulated radiation therapy (IMRT), image-guided radiation therapy (IGRT), and stereotactic body radiation therapy (SBRT) have significantly improved the precision of radiation therapy targeting, allowing for higher tumor doses while minimizing exposure to healthy tissues.
- IMRT uses dynamic multileaf collimators and inverse planning algorithms to modulate the intensity of radiation beams from multiple angles, creating highly conformal dose distributions that closely match the three-dimensional shape of the tumor. This enables dose escalation to complex tumor volumes while sparing adjacent organs at risk, which is particularly beneficial for tumors near critical structures.[22,28]
- IGRT incorporates frequent, often daily, imaging, such as CT, cone-beam CT, or MRI, immediately before or during treatment to verify and adjust patient positioning and tumor localization. This approach accounts for anatomical changes and organ motion, ensuring that the radiation is delivered precisely as planned and reducing the margin of normal tissue irradiated.[29-30] Techniques such as fiducial marker placement, respiratory gating, and four-dimensional CT further enhance targeting accuracy for tumors subject to movement.[31]
- SBRT delivers very high doses of radiation in a small number of fractions with sub-millimeter accuracy, relying on advanced immobilization, image guidance, and multiple convergent beams or arcs. This technique achieves steep dose gradients, allowing for ablative treatment of tumors while minimizing exposure to surrounding normal tissues. SBRT is particularly effective for small, well-defined tumors in the lung, liver, and spine.[31]
- Artificial intelligence-based contouring software and adaptive radiotherapy platforms further refine these techniques by automating and standardizing tumor and organ-at-risk delineation, enabling real-time plan adaptation to daily anatomical changes and supporting individualized, precision treatment.[23,27]
How well does radiation therapy work?
Radiation therapy is highly effective in treating cancer due to its ability to precisely target tumor cells using advanced techniques such as intensity-modulated radiation therapy (IMRT), image-guided radiation therapy (IGRT), and stereotactic body radiation therapy (SBRT), as well as artificial intelligence-based contouring software for improved accuracy. These technologies enable the delivery of high, conformal doses to complex or critically located tumors while minimizing exposure to surrounding healthy tissues, resulting in improved local control and reduced toxicity.[32-33]
As a standalone modality, radiation therapy achieves curative outcomes in many localized cancers (e.g., head and neck, prostate, lung, and CNS tumors) and provides effective palliation for metastatic disease. SBRT, in particular, offers exceptional local control for small, well-defined tumors, with ablative doses and steep dose gradients that spare normal tissue.[31] IMRT and IGRT further enhance precision, allowing dose escalation and margin reduction, which translates to better tumor control and fewer side effects.[28,29] AI-driven contouring and adaptive radiotherapy platforms automate and standardize tumor delineation, supporting individualized treatment and daily plan adaptation.[23,27]
When combined with other modalities, radiation therapy synergizes with chemotherapy, targeted agents, immunotherapy, and surgery to improve outcomes. Concurrent chemoradiation is standard for many locally advanced cancers, enhancing radiosensitivity and systemic control.[32] Preoperative or postoperative radiation can reduce recurrence risk, and integration with immunotherapy is an emerging strategy to leverage immune-mediated tumor eradication.[23,33]
In conclusion, the effectiveness of radiation therapy in cancer care is driven by its precise targeting capabilities, enabled by advanced imaging, planning, and delivery techniques, and further enhanced by AI-based automation. These innovations allow for individualized, high-dose treatment of tumors with minimal collateral damage, both as monotherapy and in combination with systemic and surgical approaches, making radiation therapy a cornerstone of modern multidisciplinary oncology.[32-33]
References
- Sia J, Szmyd R, Hau E, Gee HE. Molecular Mechanisms of Radiation-Induced Cancer Cell Death: A Primer. Front Cell Dev Biol. 2020;8:41. doi:10.3389/fcell.2020.00041.
- Carlos-Reyes A, Muñiz-Lino MA, Romero-Garcia S, López-Camarillo C, Hernández-de la Cruz ON. Biological Adaptations of Tumor Cells to Radiation Therapy. Front Oncol. 2021;11:718636. doi:10.3389/fonc.2021.718636.
- Hazout S, Oehler C, Zwahlen DR, Taussky D. Historical View of the Effects of Radiation on Cancer Cells. Oncol Rev. 2025;19:1527742. doi:10.3389/or.2025.1527742.
- Varzandeh M, Labbaf S, Varshosaz J, Laurent S. An Overview of the Intracellular Localization of High-Z Nanoradiosensitizers. Prog Biophys Mol Biol. 2022;175:14-30. doi:10.1016/j.pbiomolbio.2022.08.006.
- Guilbaud E, Naulin F, Meziani L, Deutsch E, Galluzzi L. Impact of Radiation Therapy on the Immunological Tumor Microenvironment. Cell Chem Biol. 2025;32(5):678-693. doi:10.1016/j.chembiol.2025.04.001.
- Boopathi E, Den RB, Thangavel C. Innate Immune System in the Context of Radiation Therapy for Cancer. Cancers. 2023;15(15):3972. doi:10.3390/cancers15153972.
- Valable S, Césaire M, Lecrosnier K, et al. Particle Therapy to Overcome Cancer Radiation Resistance: “ARCHADE” Consortium Updates in Radiation Biology. Cancers. 2025;17(9):1580. doi:10.3390/cancers17091580.
- Rückert M, Flohr AS, Hecht M, Gaipl US. Radiotherapy and the Immune System: More Than Just Immune Suppression. Stem Cells. 2021;39(9):1155-1165. doi:10.1002/stem.3391.
- Citrin DE. Recent Developments in Radiotherapy. N Engl J Med. 2017;377(11):1065-1075. doi:10.1056/NEJMra1608986.
- Sriramulu S, Thoidingjam S, Brown SL, et al. Molecular Targets That Sensitize Cancer to Radiation Killing: From the Bench to the Bedside. Biomed Pharmacother. 2023;158:114126. doi:10.1016/j.biopha.2022.114126.
- Thariat J, Hannoun-Levi JM, Sun Myint A, Vuong T, Gérard JP. Past, Present, and Future of Radiotherapy for the Benefit of Patients. Nat Rev Clin Oncol. 2013;10(1):52-60. doi:10.1038/nrclinonc.2012.203.
- Wilkinson B, Hill MA, Parsons JL. The Cellular Response to Complex DNA Damage Induced by Ionising Radiation. Int J Mol Sci. 2023;24(5):4920. doi:10.3390/ijms24054920.
- Santivasi WL, Xia F. Ionizing Radiation-Induced DNA Damage, Response, and Repair. Antioxid Redox Signal. 2014;21(2):251-259. doi:10.1089/ars.2013.5668.
- Lomax ME, Folkes LK, O’Neill P. Biological Consequences of Radiation-Induced DNA Damage: Relevance to Radiotherapy. Clin Oncol (R Coll Radiol). 2013;25(10):578-585. doi:10.1016/j.clon.2013.06.007.
- Saini S, Gurung P. A Comprehensive Review of Sensors of Radiation-Induced Damage, Radiation-Induced Proximal Events, and Cell Death. Immunol Rev. 2025;329(1):e13409. doi:10.1111/imr.13409.
- Ameixa J, Bald I. Unraveling the Complexity of DNA Radiation Damage Using DNA Nanotechnology. Acc Chem Res. 2024;57(11):1608-1619. doi:10.1021/acs.accounts.4c00121.
- Nikitaki Z, Velalopoulou A, Zanni V, et al. Key Biological Mechanisms Involved in High-LET Radiation Therapies With a Focus on DNA Damage and Repair. Expert Rev Mol Med. 2022;24:e15. doi:10.1017/erm.2022.6.
- Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and Radiation Therapy: Current Advances and Future Directions. Int J Med Sci. 2012;9(3):193-199. doi:10.7150/ijms.3635.
- Sproull M, Wilson E, Miller RW, Camphausen K. The Future of Radioactive Medicine. Radiat Res. 2023;200(1):80-91. doi:10.1667/RADE-23-00031.1.
- Beaton L, Bandula S, Gaze MN, Sharma RA. How Rapid Advances in Imaging Are Defining the Future of Precision Radiation Oncology. Br J Cancer. 2019;120(8):779-790. doi:10.1038/s41416-019-0412-y.
- García-Figueiras R, Baleato-González S, Luna A, et al. How Imaging Advances Are Defining the Future of Precision Radiation Therapy. Radiographics. 2024;44(2):e230152. doi:10.1148/rg.230152.
- Webster M, Podgorsak A, Li F, et al. New Approaches in Radiotherapy. Cancers. 2025;17(12):1980. doi:10.3390/cancers17121980.
- Dona Lemus OM, Cao M, Cai B, Cummings M, Zheng D. Adaptive Radiotherapy: Next-Generation Radiotherapy. Cancers. 2024;16(6):1206. doi:10.3390/cancers16061206.
- Richlitzki C, Manapov F, Holzgreve A, et al. Advances of PET/CT in Target Delineation of Lung Cancer Before Radiation Therapy. Semin Nucl Med. 2025;55(2):190-201. doi:10.1053/j.semnuclmed.2025.02.013.
- Jensen K, Al-Farra G, Dejanovic D, et al. Imaging for Target Delineation in Head and Neck Cancer Radiotherapy. Semin Nucl Med. 2021;51(1):59-67. doi:10.1053/j.semnuclmed.2020.07.010.
- Keall PJ, Brighi C, Glide-Hurst C, et al. Integrated MRI-guided Radiotherapy – Opportunities and Challenges. Nat Rev Clin Oncol. 2022;19(7):458-470. doi:10.1038/s41571-022-00631-3.
- Pang Y, Wang H, Li H. Medical Imaging Biomarker Discovery and Integration Towards AI-Based Personalized Radiotherapy. Front Oncol. 2021;11:764665. doi:10.3389/fonc.2021.764665.
- Mohan R. Intensity-Modulated Radiation Therapy – You Can Have Your Cake and Eat It Too! Med Phys. 2023;50 Suppl 1:74-79. doi:10.1002/mp.16100.
- Dawson LA, Jaffray DA. Advances in Image-Guided Radiation Therapy. J Clin Oncol. 2007;25(8):938-946. doi:10.1200/JCO.2006.09.9515.
- Glide-Hurst CK, Chetty IJ. Improving Radiotherapy Planning, Delivery Accuracy, and Normal Tissue Sparing Using Cutting Edge Technologies. J Thorac Dis. 2014;6(4):303-318. doi:10.3978/j.issn.2072-1439.2013.11.10.
- Bryant JM, Weygand J, Keit E, et al. Stereotactic Magnetic Resonance-Guided Adaptive and Non-Adaptive Radiotherapy on Combination MR-Linear Accelerators: Current Practice and Future Directions. Cancers. 2023;15(7):2081. doi:10.3390/cancers15072081.
- Pacelli R, Caroprese M, Palma G, et al. Technological Evolution of Radiation Treatment: Implications for Clinical Applications. Semin Oncol. 2019;46(3):193-201. doi:10.1053/j.seminoncol.2019.07.004.
- Chandra RA, Keane FK, Voncken FEM, Thomas CR. Contemporary Radiotherapy: Present and Future. Lancet. 2021;398(10295):171-184. doi:10.1016/S0140-6736(21)00233-6.





